| Issue |
EPJ Nuclear Sci. Technol.
Volume 6, 2020
Euratom Research and Training in 2019: challenges, achievements and future perspectives
|
|
|---|---|---|
| Article Number | 34 | |
| Number of page(s) | 8 | |
| Section | Part 1: Safety research and training of reactor systems | |
| DOI | https://doi.org/10.1051/epjn/2019014 | |
| Published online | 05 May 2020 | |
https://doi.org/10.1051/epjn/2019014
Review Article
Fuel fabrication and reprocessing issues: the ASGARD project
1
Nuclear Chemistry, Chalmers University of Technology, 41296 Göteborg, Sweden
2
Nuclear Research & Consultancy Group (NRG), Research and Innovation, PO Box 25, 1755 ZG Petten, The Netherlands
3
National Nuclear Laboratory, Sellafield, Seascale, Cumbria CA20 1PG, UK
4
Division of Reactor Physics, KTH, AlbaNova University Centre, 106 91, Stockholm, Sweden
* e-mail: che@chalmers.se
Received:
12
March
2019
Accepted:
4
June
2019
Published online: 5 May 2020
The ASGARD project (2012–2016) was designed to tackle the challenge the multi-dimensional questions dealing with the recyclability of novel nuclear fuels. These dimensions are: the scientific achievements, investigating how to increase the industrial applicability of the fabrication of these novel fuels, the bridging of the often separate physics and chemical communities in connection with nuclear fuel cycles and finally to create an ambitious education and training platform. This will be offered to younger scientists and will include a broadening of their experience by international exchange with relevant facilities. At the end of the project 27 papers in peer reviewed journals were published and it is expected that the real number will be the double. The training and integration success was evidenced by the fruitful implementation of the Travel Fund as well as the unique schools, e.g. practical and theoretical handling of plutonium.
© C. Ekberg et al., published by EDP Sciences, 2020
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
1 Introduction
The Strategic Energy Technology plan (SET-plan) of the European Commission identifies fission energy as an important contributor to meet long-term objectives for reduction of greenhouse gas emissions. It is argued that sustainability of nuclear power may be achieved by the introduction of the so called Gen IV systems comprising fast neutron and their associated fuel cycle facilities. The details are further described in the Strategic Research Agenda (SRA) of the Sustainable Nuclear Energy Technology Platform (SNE-TP).
A road map towards a demonstration of sustainable Generation IV systems has been defined by the ESNII task force of SNE-TP (European Sustainable Nuclear Industrial Initiative. According to this plan, it is foreseen a construction and operation of one prototype sodium cooled reactor (ASTRID) with a power of 600 MWe, two demonstration reactors using lead and gas coolant, respectively (ALFRED and ALLEGRO), a lead-bismuth cooled materials test and irradiation facility (MYRRHA), a minor actinide capable fuel fabrication pilot plant (ALFA) and other supporting facilities.
It is expected that fast neutron reactors as used in the Gen IV systems will have a breeding ratio of plutonium equal to unity, while at the same time functioning as burners of minor actinides. In order for serious demonstration on industrial scale to be achieved in the next decade requires significant R&D to be carried out in the immediate future to handle the suggestions of novel coolant technologies and advanced fuels in combination with stringent safety objectives of Generation IV systems. Such research in relevant areas is performed nationally and also in FP7 projects such as ESFR, LEADER, GOFASTR, ACSEPT, GETMAT, FAIRFUELS, FREYA and F-BRIDGE. Sadly it is not today so common that that close cooperation in international projects between the communities focussed on the different parts of a Gen IV system exist i.e. reactor, fuel and recycling communities. This could and has led to misunderstandings and sub optimisation of the different system parts e.g. that input to the fuel fabrication should be conditioned from the recycling part etc. In the case of simple MOX fuel this has been solved for plutonium and uranium fuel to a large extent but it is considerably more evident for more advanced nuclear fuels. Examples of such fuels are e.g. inert matrix fuels, nitride fuels and carbide fuels. Today, there are still considerable lack of scientific and technological maturity before any process for the manufacturing, operation and recycling of these fuels can take place.
Consistently with the above mentioned future nuclear research, the ASGARD project's main objective is to provide a structured R&D framework bridging the research on fuel fabrication and reprocessing issues. The main focus will lie on future fuels for a sustainable nuclear fuels cycle. The main problem today is to tie the recycling of the nuclear fuel to the fabrication of new fuels. Seen in this context the outline of the work on each of the fuel types will be: Dissolution (of irradiated and unirradiated fuel), Conversion and Fabrication.
These processes will be applied to the different fuel types that have been identified as possible future alternatives for the next generation of power producing reactors:
-
Oxide and CerCer/CerMet Inert Matrix Fuels
-
Nitride Fuels
-
Carbide Fuels.
In addition to this an extensive Education and Training domain was created and implemented.
2 Technical domains
As described above there are 3 technical domains in the ASGARD project. The main findings and results are given below.
2.1 Oxides and CerCer/CerMet inert matrix fuels
Dissolution and separation strategy for oxides is a fairly mature process which has been optimised and developed for Gen IV systems in several consecutive projects of which the last ones are the ACSEPT/SACSESS project. Industrial evaluation of the processes tested using genuine used nuclear fuel is ongoing. It is important to note that the successful development mentioned above is true for oxide fuels such as MOX and/or Minor Actinide containing MOX. However, for inert matrix fuels containing ceramic MgO (CerCer) or metallic molybdenum (CerMet) issues relating to their dissolution and separation has not been investigated to the same degree (or not at all). For these reasons the ASGARD project focuses mainly on the Inert Matrix Fuels (IMF) with molybdenum or magnesium oxide. The manufacturing was studied but more focus was put on the handling of the inert component in a recycling process. The issues are slightly different: for the case of MgO based fuels the bulk Mg need to be removed to prevent it entering the final vitrification and in the case of Mo based fuels the recovery of the isotopic enriched faction is important.
2.1.1 CerMet
A crucial question for the CerMet fuels is the handling of the intert matrix elements in the dissolution and subsequent separation proves. It is clear that high amounts of the intert matrix element could have a highly negative effect on the subsequent immobilisation process (impact on the stability of the waste and amount of generated waste).
The main focus has been on molybdenum based fuels where e.g. the dissolution has been comprehensively investigated. The effect of different dissolution parameters such as acid concentration and temperature on the dissolution rate as well as the influence of Fe(III) on the solubility of molybdenum have been investigated. The dissolution rates increase with increasing acid concentration, temperature and Fe(III) content. The solubility of molybdenum increase with addition of Fe(III). Unfortunately, increasing temperature and nitric acid concentration leads to increased precipitation.
To clarify whether the Mo matrix forms mixed species with actinides upon dissolution in HNO3, mixed 98Mo and 90Zr (IV) (as analogue for Pu (IV)) solutions have been measured by electrospray ionization mass-spectrometry (ESI-MS). The formation of mixed Mo-Zr species in nitric acid was observed. The mixed species relative abundance of Mo decreases with decreasing Zr concentration and decreasing nitric acid strength in the samples. The formation of poorly soluble mixed Mo-Zr compounds could affect the reprocessing procedure. A small fraction of molybdenum in solution is present in the oxidation state +5 [1].
The investigation of solutions containing Mo plus Eu(III) (as Am(III) analogue) at 0.5, 1 and 3 mol/L HNO3 were successfully performed. In these experiments it was shown that several mixed complexes are formed such as MoO2Eu(NO3)(OH)3 + (H2O)n. It is very likely that other mixed complexes also existed in the solution at this time. It is highly likely that these mixed Mo(actinide) complexes will have a significant impact on the subsequent separation process since they may hinder the actinide extraction and recovery. ATR-FTIR was used to elucidate structural information on the solution species using two pure molybdenum samples in 0.5 and 3 mol/L HNO3.
Separation of strontium from molybdate solution by using different absorbents has been tested; the most prospective one was Ba(Ca)SO4 which was selected for future testing. A weight distribution ratio of Dg > 250,000 mL/g was found for this material, which is a value suitable for the design of a process for quantitative separation of Sr from the concentrated solution of molybdenum. A composite absorber using a polyacrylonitrile binding matrix was precipitated using Ba(Ca)SO4. In dynamic column experiments, it was shown that the Ba(Ca)SO4–PAN absorber is very efficient for the removal of strontium from simulated molybdate solution [2].
Three types of fresh fuels (5, 10, 25 and 40 wt% of CeO2, UO2, PuO2 resp.) in molybdenum matrix have been fabricated by powder metallurgy method and fully characterized [3,4]. Dissolution experiments on mixed Mo/CeO2 pellets have been performed in 20 and 100 mL 1 mol/L HNO3 without Fe(III) or containing 1 equivalent of Fe(III) per equivalent of Mo at room temperature. Generally is is possible to say that iron presence increase the rate of dissolution of molybdenum at the same time as the dissolution rate of Ce is unchanged. In the absence of Fe(III) a pale precipitate appears after about 100 h, which corresponds to a drastic drop in molybdenum concentration [5,6] The setup dissolution conditions was also successfully applied to the dissolution study of actinides fresh fuels.
Another option than separation by dissolution could be a thermal treatment of the material. Such a treatment is based on the fact that molybdenum is oxidized in air at temperatures from 400 °C and the resulting MoO3 sublimes at 800 °C. Although still not efficient enough the proof of concept was successfully tested using pure molybdenum and CeO2/Mo materials. Further optimisation will be needed for practical use.
In order to provide a good fabrication process zinc stearate is used as additive during pellets production, i.e. the dissolution solution will contain Zn(II). Therefore the extraction of Zn(II) from 0.1 to 3 mol/L HNO3 into TBP, DMDOHEMA and TODGA solvents was studied. Fortunately, Zn(II) was shown not to be extracted in PUREX and DIAMEX type processes.
Real irradiated (Pu0.8Am0.2)O2 in Mo matrix from HFR Petten [7] was studied with respect to its dissolution behaviours. The inert matrix was dissolved in 4 mol/L HNO3 at ambient temperature. The dissolved material was then removed. Dissolution of the actinide oxide material in boiling 8 mol/L HNO3 with addition of HF or Ag(II) was not fully successful; a black residue remained [8].
2.1.2 CerCer
The initial study was performed in fresh MgO pellets in 2.5 mol/L HNO3 at 30 °C. It could be concluded that that agitation speed has no effect on dissolution rate, indicating that the dissolution rate is controlled by the dissolution reaction. It was also found that there was no effect on the dissolution rate of the acid volume used.
Based on these experiments a mechanism of the MgO dissolution has been proposed [9]. This mechanism involves a two-stage reaction equation based on XRD measurements and literature review. It was concluded from microstructural investigations on pellets subject to 15 hours of 2.5 mol/L HNO3 at 30 °C that there was a heterogeneous development of the pellet surface. The normalization of the dissolution rates to the geometrical surface area showed varying dissolution rates after different reaction times. The consideration of the additional pellet surface obtained by the development of holes resulted in a dissolution rate of approximately 0.02 g s−1 m−2.
In order to simulate plutonium content in the MgO matrix fresh CeO2 containing pellets were prepared and fully characterized. Microstructural investigations of the pellets show a heterogeneous distribution of CeO2. From a detailed dissolution study of these pellets it became clear that the separation of the actinide bearing phase could be separated during the dissolution stage.
2.1.3 Oxides
The oxide fuels study focuses mainly on the methods for conversion from solution to suitable oxide precursors; different methods have been investigated:
-
various sol-gel routes
-
methods for co-conversion of actinides by impregnation of solid matrixes
-
radiation and photochemical techniques for conversion of actinides to solid matrixes.
The internal gelation method was used for synthesis of pure uranium oxide, and uranium/neodymium oxide microspheres. During facrication the Nd content was varied between (5–40%) [10]. After a characterization, the particles were thermally treated under reducing conditions at 1300 °C and 1600 °C. In order to characterise the pellets in more detail SEM/EDX and X-ray powder diffraction (XRD) was used. The XRD data was then further used to elucidate lattice parameters. It could then be verified that the internal gelation synthesizing route can be used to fabricate the equilibrium solid solutions of the sensitive UO2/Nd2O3 system.
A new method called Complex Sol-Gel Process (CSGP) [11,12] and Double Extraction Process − simultaneously extraction of water and nitrates by Primene was investigated for synthesis of uranium dioxide microspheres doped by surrogates of Pu and Minor Actinides (MA). IT was tested for fabrication of uranium oxide microspheres doped up to 40 wt% of Nd. During the investigation all included fabrication steps were investigated. Some focus was put on the thermal heating which required a detailed study (TG-DSC) to minimize cracks in the sintered microspheres. ICP-MS, SEM, EDS and weight analysis was used to characterise the gels and oxides. EDS mapping analysis confirmed homogeneity distribution of all elements U and Nd (even 40%) in whole volume of microsphere. It was confirmed that neodymium was built. In the UO2 structure using X-ray fluorescence (XRF) analysis.
Regarding solid–liquid extraction, various parameters were investigated to maximise sorption onto Amberlite IRC-86 and Lewatit TP-207. The resins were loaded with UO22+ and Nd3+ [13] and the temperature influence and the effect of the pH on the adsorption was investigated. The adsorption kinetics of UO22+, Nd3+ and a mixture of both ions was studied. The latter studies revealed a significantly faster adsorption of Nd3+ compared to UO22+. After about 18 h the adsorption of both UO22+ and Nd3+ reaches equilibrium. An exchange of UO22+ and Nd3+ is observed for mixtures for contacting times >18 h. The effect of pH on the adsorption is profound while there is a very small effect of changing the temperature. A considerable decrease in adsorption was observed at pH < 3. A solution to be able to keep the pH high enough without introducing additional chemical elements is to use acid-deficient uranyl nitrate (ADUN) solutions. The thermal behaviour has been studied by TG-DSC. The thermal treatment of the particles in air was studies and the products were characterized by SEM/EDX and XRD techniques.
Radiation-induced preparation of nuclear fuels seems to be very promising fabrication route; in ASGARD methods utilizing formates as OH radical scavenger and UV light has been applied for preparation of uranium and thorium hydroxides [14,15]. Both UV and gamma assisted precipitation was used and the precipitates were investigated using EXAFS and XRD. Pellets have been prepared from these synthetized materials by powder metallurgy method, sintered at 1300–1600 °C and characterized by XRD, porosimetry and SEM. No binder or lubricant was mixed with the starting material powder (only stearic acid was used as a die-wall lubricant) and sintered pellets reached a density of 90–97% TD.
2.2 Nitride fuels
Due to a higher actinide density and a combination of high thermal conductivity with high melting temperature nitride fuels constitute a better performing alternative to oxide fuels. The higher melting point of the nitrides are particularly important in the context of transmutation in Gen IV reactors, since the addition of minor actinides to the fuel is detrimental for reactivity feedbacks. Since it is possible to experience clear increases in fuel temperature transients the nitrides are better able to handle this due to their larger margin to failure. Important aspects to consider is the fabrication routes to minimise impurities of oxides, metals or carbides in addition to the need to recycle the 15-N used in the fuel production to minimise the production of 14-C during reactor operation. There are some concerns related to the solubility rate of inert matrix nitride fuels, such as (Pu,Zr)N, since the rate for dissolution of ZrN has been measured to be considerably slower than that of UO2 (albeit much faster than for PuO2) [16]. Moreover, in the Bora-Bora experiment, it was observed that insoluble PuO2 inclusions formed during irradiation of (Pu,Zr)N featuring oxygen impurities [17]. Hence, it is of interest to determine whether inert matrix nitride fuels can be fabricated with sufficiently low impurity levels to avoid issues related to dissolution performance. Furthermore, there is a need to enrich the nitrogen used for production of nitride fuels in N-15 [18]. This is a costly process, which makes in necessary to reduce costs for enrichment, as well as to establish a route for recycle of N-15 during reprocessing of irradiated nitride fuel. The aforementioned matters are addressed within the nitride domain of ASGARD. In particular the following issues are investigated:
-
Dissolution performance of as-fabricated and irradiated inert matrix nitride fuel.
-
Manufacture of inert matrix nitride fuel with controlled carbon and oxygen impurity levels.
-
Cost for enrichment of N-15, reduction of N-15 losses during fuel fabrication and N-15 recovery during reprocessing.
2.2.1 Dissolution performance
Within the FP5 CONFIRM project, (Pu_0.3,Zr_0.7)N pellets were produced by PSI for irradiation in HFR. A 170 full power day tailored spectrum irradiation took place in Petten during 2007, yielding 10% fission in actinides. Post irradiation examination revealed a fuel in good condition, with a closed fuel-clad gap, moderate cracking, 9% swelling, low release of xenon and high release of helium. As part of ASGARD, dissolution tests were carried out of irradiated CONFIRM fuel. Dissolving pellets in 8 M boiling nitric acid, the dissolution proceeded from the centre (low burn-up) part of the pellet, leaving a black residue at the high burn-up rim of the pellet. The composition of the residue remains to be determined. Possibilities include plutonia or more likely zirconia inclusions forming during irradiation. It should be mentioned that no such inclusions were present in the as-fabricated fuel. Moreover, dissolution tests of archive (sintered) powders from the CONFIRM manufacturing campaign showed that these dissolved completely within 8 hours in 4–10 M boiling nitric acid.
The aforementioned data indicate that even though up to 0.35 wt% oxygen could be accommodated as a soluble compounds in fresh inert matrix (Pu,Zr)N fuel, the precipitation of insoluble oxide phases during irradiation may still occur. Therefore, it is important to establish routes for minimising the oxygen content during manufacture of this fuel. This can however not be done on the expense of introducing too much carbon, since carbo-nitride fuels are known to have issues related both to fuel-clad chemical interaction (FCCI) and formation of organic residues during reprocessing.
2.2.2 Manufacture of inert matrix fuels
From the industrial perspective, the most straight-forward route for manufacture of nitride fuels is carbo-thermic nitriding of oxide powders. This route was investigated in detail within the CONFIRM project. Later, collaboration between JAEA and KTH (co-funded by ASGARD) showed that low levels of both carbon and oxygen can be achieved by combining manufacture of PuN using carbo-thermic nitriding of PuO2 with hydriding/nitriding of Zr metal. Alternative routes for manufacture of inert matrix nitride fuels have been investigated. Due to licencing limitations the work is divided into investigation of mechniams using “inactive” substances such as U and Zr and more active substances involving Pu. At KTH, UN powder is produced by hydrating/nitriding of uranium metal. If carried out in a glove box, this process may yield extremely pure UN, with less than 50 ppm UO2. The powders are not fabricated in a glove box, resulting in oxygen impurities ranging from 800 to 1600 ppm weight. Pellets produced from these powders using spark plasma sintering under a reducing atmosphere at T = 1650 °C contain between 500 and 1200 ppm oxygen [19]. Albeit higher than achievable in an ideal process, these values meet the 1500 ppm criterion for avoiding issues with PCCI suggested by Rogozkin for (U,Pu)N fuels [20]. First attempts in manufacturing ZrN along the same principles so far have resulted in materials with considerably higher oxygen impurities, indicating that manufacture of ZrN needs to be carried out under a protected atmosphere.
The use of wet routes for manufacture of TRU bearing nitrides is attractive, as it may allow to avoid dust formation. (Pu,Zr)N pellets have been manufactured using the sol–gel route. Here, the carbo-thermic nitriding of zirconia microspheres poses a special challenge in terms of reducing impurities to target levels. Elemental analysis of these pellets will be carried out in the latter part of 2015. At this point it is, however, clear that the carbon content of the produced pellets is too high compared to the initial plan due to both not complete nitridation but also contamination from the oven used. A good aspect though is that SEM analysis show that there is no blackberry structure left in the sintered pellets.
2.2.3 N-15
N-14, the predominant isotope of natural nitrogen (99.7%) forms C-14 during irradiation due to (n,p) reactions. The minute presence of nitrogen in oxide fuels (and corresponding C-14 formation) has already mandated installation of means for carbon capture and immobilisation in Sellafield off-streams. Therefore, it has been suggested that nitride fuels for fast reactors should be enriched in N-15 [21]. However, the current cost for N-15 is larger than that of manufacture of MOX fuel. Hence, the ASGARD project includes development of methods for reducing the cost for N-15 enrichment, minimisation of nitrogen losses during manufacture of nitride fuels, as well as provisions for recycle of N-15 from used nitride fuel.
A facility for N-15 enrichment using the Nitrox method under pressure has been built. The Nitrox method is based on isotopic exchange between nitric acid and nitrogen oxides according to: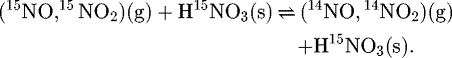
ASGARD experiments have shown that the flow rate of nitric acid in the column for N-15 separation can be increased by 50% by operating at a pressure of 1.2 bar.
In the product refluxer of the isotope separation plant, nitric acid is converted into nitrogen oxides by reaction with sulfur dioxide:
whereas in the waste refluxer, nitrogen oxides are converted into nitric acid by reaction with oxygen and water:
Since more than 50% of the cost for N-15 enrichment in current facilities is due to the feed of sulphur dioxide, the conversion of sulphuric acid from the product reflux to sulphur dioxide may allow a significant cost reduction. To this end, the efficiency of several catalysts for the aforementioned reaction was investigated. It was shown that α-Fe2O3 may provide higher conversion rates than more expensive alternatives. Using an Incolloy 800 reactor at 850 °C it was possible to reach a conversion rate of 58% for reduction of sulfuric acid to sulfur dioxide
A gas conserving method for manufacture of U2N3 was further developed based on hydriding/nitriding of uranium metal. Gas consumption measurements conducted on-line during the fabrication process shows that the uptake of nitrogen supplied to the process can be made nearly complete. A caveat with this approach is that oxides deriving from reprocessing of spent fuel would have to be converted to metals before nitriding can take place.
Finally, a process for recovery of N-15 following conversion of UN to an oxide by exposure to steam at 500 °C has been verified. As a result of this treatment it was possible to obtain a pure stream of ammonia (enriched in N-15) leaving a dry uranium oxide powder suitable for dissolution in nitric acid.
2.3 Carbides
Carbides have in many cases similar advantages as the nitrides, i.e. their high thermal conductivity and high melting point. Thus also the carbide performance ensure increased power-to-melt margin and that fatter (more economic) pins are facilitated. Sadly there is a potential issue relating to the potential for unacceptable fuel/clad mechanical interaction (FCMI). This is typically due to the high swelling and low plasticity of dense carbide materials. In addition the carbide powders are pyrophoric which complicates the production procedure of the pellets and the reprocessing is complicated due to potential hydrocarbon complexants affecting the distribution in the solvent extraction process. Some of these are also flammable which cause additional issues. Other important issues with carbides is the recycling process and then more specifically the dissolution. In principle two different routes are foreseen: either direct dissolution or pre oxidation and then use of the current recycling technology.
2.3.1 Fuel/clad interactions
Studies using the CARTRAF code provided a parameter sensitivity analysis to ascertain the effect of deviations in the reference pin design [22] and their potential performance benefits, particularly any which conform to the objective of reducing fuel swelling whilst maintaining good thermal properties. During the course of this work, it became apparent that the fuel swelling is most sensitive to the fuel temperature. Consequently, deviations in the pin design that significantly alter the fuel temperature also altered the fuel swelling. Fuel temperatures and, therefore, fuel swelling were most sensitive to the peak mass rating, the initial radial gap size, pellet outer radius and the upper plenum volume. Higher temperatures result in larger gas release, which yields lower fuel swelling (Fig. 1).
For Sphere-Pac fuel the important variables were bed load, inter-particle necking and thermal conductivity of the packed bed using the SPACON code. A high ratio between small and large particles gave the most optimum results.
 |
Fig. 1 Fuel swelling (level 4) for the optimized and reference carbide fuel pin designs. |
2.3.2 Cabide powder pyrophoricity
There are significant hazards associated with using powdered uranium and plutonium carbide material including the pyrophoric nature in the presence of oxygen [23]. Specialist facilities have allowed the oxidation of freshly milled powder to be heated under controlled atmosphere. The material ignition profile has shown a rapid increase in temperature and the material glows then in a second stage the material sparks with a large increase in the volume of material. Bed depth profiling using PXRD has supported an oxidation mechanism to U3O8 via UO2. Models of this process have been developed demonstrating the importance of gas diffusion through the initial oxide layer and heat transfer from the powder bed (Fig. 2).
 |
Fig. 2 Ignition near 100 °C of 6 mm thick UC powder bed (3 grams) (left) pictures during ignition; (right) temperature profile as the base temperature is increased. |
2.3.3 Carbide fuel recycling
There are difficulties with reprocessing spent (U,Pu)C fuel. If the material is processed according to current industrial methods then a liquor feed containing organic molecules would be produced that can interfere with U and Pu solvent extraction and can impact on downstream high active liquor processing plants. There is a need to understand the reaction kinetics and identify the organic species produced and then attempt to reduce their formation or destroy them once formed.
The spent (U,Pu)C could undergo a pre-oxidation step although experience has shown that separation of insoluble plutonium rich phases can occur resulting in difficulties during the following dissolution step. In addition, there are uncertainties in the how certain fission products will behave during high temperature pretreatment potentially leading to an increase in the amounts of highly radioactive fission products entering the off-gas stream.
For the peroxidation step the use of CO2 has been explored to control the oxidation as far as UO2 and preventing PuO2 phase separation. Thermodynamic calculations were supported by experimental evidence that demonstrated material oxidized by CO2 readily dissolved without significant PuO2 insoluble residues and without the production of soluble organics (Fig. 3). The use of CO2 as an oxidant may be prohibitive if 14C capture is to be used within the off-gas treatment plant.
Direct dissolution of arc melted carbide ingots (∼1 g) and large (70 g) UC pellets have been dissolved and the effects of temperature, [HNO3] and [HNO2] on the reaction kinetics. The dissolution mechanism is very complicated and the dissolution rate appears to be strongly correlated with the temperature of the reaction and the level of HNO2 within the system. The stability of HNO2 at high temperatures appears to limit any increase in the reaction rate beyond 80 °C (Fig. 4).
A detailed analysis of the organic species left in solution, once all of the UC dissolved, confirmed previously unidentified nitrated unsaturated organic molecules. Using a combination of ion and liquid chromatography, NMR, IR and UV/vis spectroscopy and mass spectrometry a clearer picture of the complex mixture was determined (Fig. 5).
 |
Fig. 3 Dissolution of (U0.8,Pu0.2)C with (left) and without (right) pre-oxidation in CO2 at 1000 °C. |
 |
Fig. 4 The effect of temperature on the total carbon dissolved throughout the 70 g UC pellet (right) dissolution. [HNO3]ini = 8 M (e.g. UC50 is 50 °C and UC110 = 110 °C). |
 |
Fig. 5 Carbon speciation during UC dissolution. |
3 Education and training
The Education and Training Domain is focussed on the stimulation of the exchange of knowledge and practical experience among the nuclear fuel community and increase the experience of future researchers. As future researches the main target group was MSc and PhD students. However, also teachers and other members of the community will benefit from ASGARD activities and measures in area of education/training and mobility. The base for the courses was the background and the obtained knowledge in the different technical domains. A special Winter School in nuclear fuel manufacturing was given jointly between ASGARD and the FP-7 projects FAIRFUELS and CINCH in January 2013 in Petten (NL). A special course in industrial manufacturing techniques was given by one of our industrial partners, Westinghouse. Special emphasis was put on safety aspects related to dissolution, conversion, reprocessing and fuel fabrication under normal and accident conditions. Another Winter School has been held in Stockholm in January 2014 was focussed on fuel characterization and isotope separation Since the ASGRAD project is dealing with the practical handling of substantial amount of radioactive material a continuous feed-back and eventual improvements with regard to safety and materials will be established and implemented throughout the project.
A school directed to the hands on and theoretical studies of the chemistry of plutonium was organised at Chalmers as a joint venture by ASGARD and ACSEPT.
Joint presentations with the ACSEPT project were made during the ATALANTE conference in September 2012 where ACSEPT handled a session on separations and ASGARD one session on actinide materials chemistry. The first ASGARD International seminar was given at the 17th International Radiochemistry Conference RadChem in May 2014.
A highly successful travel and mobility support was developed and used during the project under DM1 named Travel Fund. The aim was to allow young scientists, students and trainers to disseminate and network into the community, as well as have access to relevant facilities. 24 grants were approved, of which 5 were for mobility to other laboratories, 1 for a trainer mobility and 18 for summer/winter school and conference participation.
The outreach of ASGARD project was measured in numerous publications in peer-review journals, as well as conferences and public media. As a result, more than 50 scientific publications (of which 27 is in peer reviewed journals) and 14 press releases have been achieved so far. This is a number which is expected to increase since some work is still not yet published.
4 Conclusions
All in all the ASGARD project was a clear success in all its objectives. The education and training domain was highly successful in making both young science staff exchange as well as giving courses and training in very diverse fields such as separation science and hands on plutonium handling. The technical domains advanced the field of advanced fuel fabrication considerably in all domains including reaching additional industrial potential for some fuel types.
Acknowledgments
The research leading to these results has received funding from the European Atomic Energy Community's Seventh Framework Programme FP7/2007-2011 under grant agreement n°295825e NNL would also like to thank the Nuclear Decommissioning Authority and the NNL Signature Research program for financial support
References
- M. Cheng, M. Steppert, C. Walther, On the dissolution behavior of new Mo fuel matrices for Generation IV Reactors, in GDCh-Tagung 2013 Darmstadt, September 1st-4th 2013, Darmstadt, Germany (2013) [Google Scholar]
- K.V. Mareš, J. John, F. Šebesta, in Booklet of Abstracts, 17th Radiochemical Conference, 11-16 May 2014, Mariánské Lázně , edited by V. Bečková (2014), p. 383, ISBN 978-80-01-05504-5 [Google Scholar]
- E. de Visser-Týnová, Asgard deliverable D 2.1.1–Report on fabrication of Mo-based inert matrix fuels with UO2 and PuO2 as actinide compound, 2014 [Google Scholar]
- E.L. Ebert, A. Bukaemskiy, F. Sadowski, F. Brandt, M. Cheng, M. Steppert, C. Walther, G. Modolo, D. Bosbach, in First joint workshop on f-element chemistry , 28th–30th April 2014, University of Manchester, UK [Google Scholar]
- E.L. Ebert, A. Bukaemskiy, F. Sadowski, F. Brandt, G. Modolo, D. Bosbach, in 17th Radiochemical Conference, 11–16 May 2014, Marianske Lazne, Czech Republic, Booklet of Abstracts (2014), p. 383, ISBN 978-80-01-05504-5 [Google Scholar]
- E.L. Ebert, M. Cheng, M. Steppert, C. Walther, G. Modolo, D. Bosbach, in 17th Radiochemical Conference, 11–16 May 2014, Marianske Lazne, Czech Republic, Booklet of Abstracts (2014), p. 354, ISBN 978-80-01-05504-5 [Google Scholar]
- E. D'agata, J.M. Lapetite, F.C. Klaassen, S. Knol, C. Sciolla, J. Somers, A. Fernandez-Carretero, J.M. Bonnerot, F. Delage, Prog. Nucl. Energy. 53 , 748 (2011) [CrossRef] [Google Scholar]
- G. Menard, E. de Visser-Týnová, in ACTINIDES 2013 , Karlsruhe, Germany [Google Scholar]
- E.L. Ebert, A. Bukaemskiy, F. Sadowski, F. Brandt, G. Modolo, D. Bosbach, in 17th Radiochemical Conference, 11–16 May 2014, Marianske Lazne, Czech Republic, Booklet of Abstracts (2014), p. 383, ISBN 978-80-01-05504-5 [Google Scholar]
- C. Schreinemachers, A.A. Bukaemskiy, M. Klinkenberg, S. Neumeier, G. Modolo, D. Bosbach, in 17th Radiochemical Conference, 11–16 May 2014, Marianske Lazne, Czech Republic (2014) [Google Scholar]
- A. Deptuła, M. Brykala, M. Rogowski, T. Smolinski, T. Olczak, W. Łada, D. Wawszczak, A.G. Chmielewski, K.C. Goretta, in 2014 MRS Spring Meeting & Exhibit , April 21–25, 2014, San Francisco, California [Google Scholar]
- M. Brykala, A. Deptula, M. Rogowski, Ch. Schreinemachers, G. Modolo, in 13th Information Exchange Meeting − Actinide and Fission Product Partitioning and Transmutation , Seoul, South Korea, September, 23–26th, 2014 [Google Scholar]
- R. Middendorp, C. Schreinemachers, S. Neumeier, G. Modolo, D. Bosbach, in 17th Radiochemical Conference , 11–16 May 2014, Marianske Lazne, Czech Republic, p. 383, ISBN 978-80-01-05504-5 [Google Scholar]
- T. Pavelková, V. Čuba, F. Šebesta, J. Nucl. Mater. 442 , 29 (2013) [CrossRef] [Google Scholar]
- T. Pavelková, V. Čuba, F. Šebesta, Booklet of Abstracts, in 17th Radiochemical Conference , 11–16 May 2014, Mariánské Lázně, p. 375, ISBN 978-80-01-05504-5 [Google Scholar]
- H. Kleykamp, J. Nucl. Mater. 275 (1999) [CrossRef] [Google Scholar]
- B.D. Rogozkin et al., Atomic Energy 109 , 6 (2011) [Google Scholar]
- J. Wallenius, S. Pillon, in Proc. AccApp/ADTT, ANS , 2001 [Google Scholar]
- K. Johnson, J. Wallenius, M. Jolkkonen, J. Nucl. Mater. (2015), Submitted to publication [Google Scholar]
- B.D. Rogozkin et al., Atomic Energy 95 , 835 (2003) [CrossRef] [Google Scholar]
- J.E. Till et al., Nucl. Technol. 37 , 328 (1978) [CrossRef] [Google Scholar]
- K.R. Krummerer, J. Nucl. Mater. 124 , 147 (1984) [CrossRef] [Google Scholar]
- R.G. Snowden et al., Behaviour of carbides in hydrogen and oxygen, in Carbides in Nuclear Energy (Macmillan & Co, London, 1964), Vol. 1 [Google Scholar]
Cite this article as: Christian Ekberg, Teodora Retegan, Eva De Visser Tynova, Mark Sarsfield, Janne Wallenius, Fuel fabrication and reprocessing issues: the ASGARD project, EPJ Nuclear Sci. Technol. 6, 34 (2020)
All Figures
 |
Fig. 1 Fuel swelling (level 4) for the optimized and reference carbide fuel pin designs. |
| In the text | |
 |
Fig. 2 Ignition near 100 °C of 6 mm thick UC powder bed (3 grams) (left) pictures during ignition; (right) temperature profile as the base temperature is increased. |
| In the text | |
 |
Fig. 3 Dissolution of (U0.8,Pu0.2)C with (left) and without (right) pre-oxidation in CO2 at 1000 °C. |
| In the text | |
 |
Fig. 4 The effect of temperature on the total carbon dissolved throughout the 70 g UC pellet (right) dissolution. [HNO3]ini = 8 M (e.g. UC50 is 50 °C and UC110 = 110 °C). |
| In the text | |
 |
Fig. 5 Carbon speciation during UC dissolution. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


